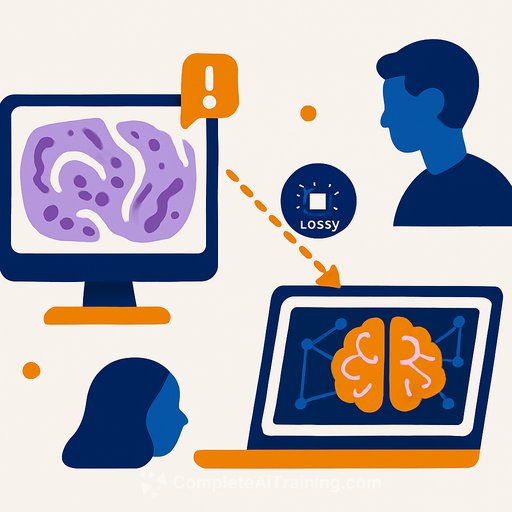
Lossy DICOM Conversion and Its Impact on AI Performance in Pathology
The digitization of pathology glass slides is advancing, with the DICOM format emerging as a preferred standard to ensure long-term accessibility and interoperability of whole slide images (WSIs). However, many scanners produce proprietary file formats and rely on conversion tools to generate DICOM images. These conversions often involve lossy compression steps that can subtly alter image data, raising concerns about their impact on downstream tasks such as artificial intelligence (AI)-based diagnostics.
Why DICOM Matters in Pathology
DICOM (Digital Imaging and Communications in Medicine) is the established format in radiology for storing diagnostic images, integrating patient metadata, and supporting anonymization. In pathology, DICOM extensions specifically address the storage of tiled, pyramidal WSIs, enabling efficient access and integration across systems. Open and interoperable formats like DICOM are crucial for collaborative diagnostics, long-term data reuse, and AI research.
Despite these advantages, most pathology scanners do not natively produce DICOM images. Instead, vendor-specific proprietary formats dominate, necessitating conversion tools. These tools often decompress the proprietary images and recompress them into DICOM format, which is not always a lossless process.
Assessing Image Quality After DICOM Conversion
In an investigation, MRXS files (a proprietary Mirax Scan format) depicting bladder, ovarian, and prostate tissue were converted to DICOM using two converters: a vendor-specific tool (SlideMaster by 3DHistech/Sysmex) and an open-source tool (wsidicomizer). Both used baseline JPEG compression but with differing parameters.
Visual inspection by pathologists showed no perceptible differences between original and converted images, even at diagnostic resolutions. However, quantitative analysis using the Structural Similarity Index (SSIM) revealed differences with values ranging roughly from 0.85 to 0.96, indicating that images are not identical. SlideMaster generally achieved higher SSIM scores than wsidicomizer, especially at lower magnifications.
One notable factor is the handling of overlapping tiles in MRXS files. Original files store tiles with overlap, requiring stitching during viewing. DICOM files, in contrast, contain non-overlapping tiles. Differences in stitching and tile extraction methods contributed to variations between the original and converted images, although these were not visible to the naked eye.
Can AI Detect the Differences?
Small pixel-level changes, even those imperceptible to humans, can affect AI models, particularly convolutional neural networks (CNNs) and foundation models. In this study, AI models were trained to distinguish between original MRXS images and their DICOM-converted counterparts. Results showed that AI could differentiate them with up to 99.5% accuracy in most cases.
Moreover, AI models trained on original images sometimes experienced significant performance differences when tested on converted images, especially in scenarios with limited training data. This suggests that even subtle image alterations from lossy conversion can impact diagnostic AI outcomes.
Implications for Diagnostic Use and AI Development
These findings emphasize the need to thoroughly (re-)evaluate all image processing and AI algorithms when switching to DICOM-converted WSIs for diagnostics. Since images are not identical post-conversion, regulatory compliance and clinical validation must be ensured with converted datasets before deployment.
Nonetheless, the DICOM format remains a promising solution for interoperability and future-proofing pathology imaging. Initial AI training experiments with converted images did not show systematic performance degradation, indicating that DICOM adoption is feasible with proper validation.
Key Takeaways
- Most pathology scanners produce proprietary formats requiring conversion to DICOM, often involving lossy compression.
- Visual quality differences after conversion are negligible, but quantitative metrics reveal measurable changes.
- AI models can detect differences between original and converted images, which may affect AI diagnostic performance, especially with limited data.
- Diagnostic use of DICOM-converted images demands rigorous validation of all algorithms and workflows.
- DICOM offers a viable path to standardization and interoperability in digital pathology.
For researchers and professionals working with AI in pathology, understanding and addressing the impact of image conversions is vital. Ensuring that AI models are trained and validated on the exact image formats intended for clinical use will safeguard diagnostic accuracy and reliability.
Learn more about AI model training and medical imaging standards at Complete AI Training.
Your membership also unlocks: